Effect of hyaluronic and poly-L-lactic acid-based fillers on Сollagen synthesis

The widespread use of fillers in modern cosmetology makes it necessary to thoroughly understand the processes occurring in the skin during their introduction: only then will the result be predictable and the procedure will be safe for patients. Here is the result of comparative in vitro and in vivo studies of the effect of two types of fillers: hyaluronic and poly-L-lactic acid based.
Introduction
Skin aging is a dynamic process that includes clinical and biological phenomena. The former include internal manifestations of aging, which are similar in all internal organs. The latter include external aging (photoaging), which occurs under the influence of mainly ultraviolet radiation . Under the influence of internal and external factors, the structural integrity of the dermis is disturbed, and enzymatic breakdown of collagen is initiated under the action of metalloproteinases and as a result of age-related decrease in the number of active fibroblasts .
These changes are visually manifested as wrinkles, tissue atrophy, elastosis, discoloration and loss of tissue volume . Thus, from the point of view of the aging process, it is not advisable to correct the defect one time and permanently, it is preferable to inject fillers as needed, eliminating signs of aging as they appear.
Currently, there are many fillers used in daily clinical practice for cosmetic and medical indications. They can be divided into preparations of temporary, semi-permanent and permanent (permanent) action, as well as according to their composition. The basis of fillers most often consists of: collagen, hyaluronic acid (HA), poly-L-lactic acid (PLLA), calcium hydroxyapatite and polymethylmethacrylate.
Hyaluronic acid is a glycosaminoglycan, it is a natural component of the extracellular matrix of the dermis . It affects cell behavior and metabolism , increases skin hydration and stimulates fibroblast proliferation by activating the expression of collagen type I and matrix metalloproteinase-1 by fibroblasts. In addition, hyaluronic acid affects the organization of actin cytoskeleton, determines the shape and orientation of fibroblasts . Poly-L-lactic acid is a biocompatible, biodegradable, immunologically inert synthetic polymer. After injection of PLLA-based filler, as its microparticles decompose, an inflammatory reaction occurs, which promotes the formation of fibrous connective tissue and neocollagenesis, resulting in gradual restoration of lost tissue volumes. In addition, PLLA increases the activity of fibroblasts and stimulates collagen synthesis. This result is not a consequence of the drug action as such, but of the body’s reaction to its administration.
However, at the molecular and cellular level, the processes responsible for these results are still poorly understood. The present study was designed to evaluate cell viability and proliferation as well as type I collagen expression after injections of hyaluronic and poly-L-lactic acid-based fillers using human skin fibroblasts. In addition, histologic aspects and in vivo expression of type I collagen were studied in a laboratory animal model.
Materials and methods
The study consisted of two parts. The first was an in vitro experiment, the main purpose of which was to evaluate the effect of hyaluronic and poly-L-lactic acids on human skin fibroblast culture in terms of cell proliferation and viability, as well as the formation of type I collagen. The second part was an in vivo study aimed at quantitative assessment of skin histological parameters after HA and PLLA application, including the distribution of biomaterials between collagen fibers and the presence of inflammatory cells, as well as estimation of the amount of type I collagen.
IN VITRO study. Cell culture
Human skin fibroblasts were obtained from explants taken during mammoplasty and abdominoplasty from three different female donors (age: 42 to 57 years).
The cellular phenotype of the fibroblasts was confirmed by immunofluorescence for antibodies to alpha-smooth muscle actin, type I collagen, and vimentin (positive), and negative for pancytokeratin cocktail (AE1/AE3 antibody) (Fig. 1).
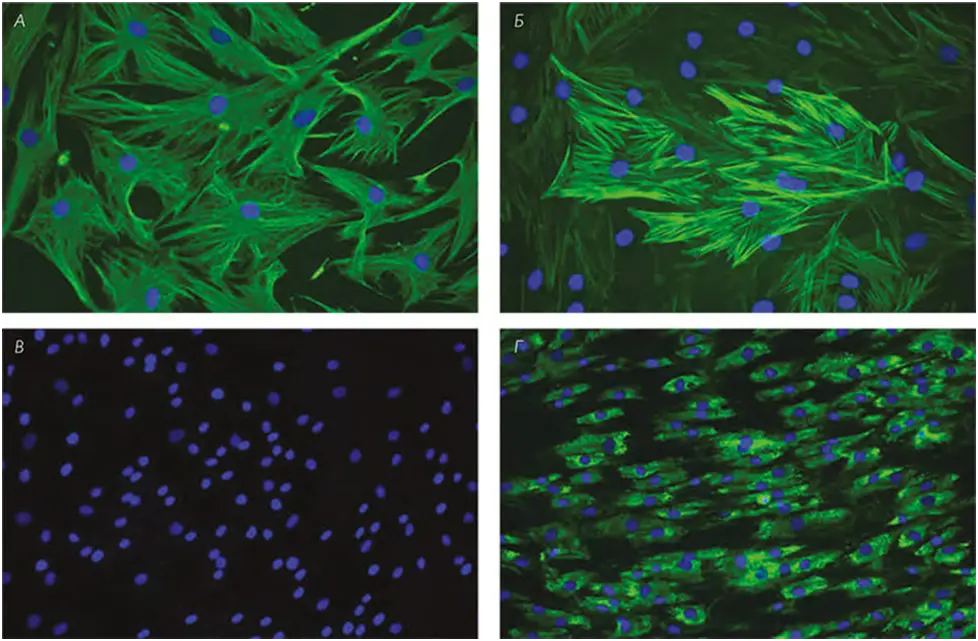
FIG. 1 A, immunolocalization of vimentin; B, alpha smooth muscle actin; C, AE1/AE3; D, type I collagen (green) in skin fibroblast culture. DNA staining (DAPI blue). Scale: A and B = 100 μm; C and D = 50 μm.
Analysis of cell proliferation and viability
The effect of fillers on human skin fibroblasts was assessed by analyzing cell proliferation and viability, as well as type I collagen expression. Cells were grown in 24- and 96-cell plates at an initial density of 110 cells/mm2 per cell. After 24 h, the medium was replaced with the same medium (C, control) or supplemented with 0.5, 1.0, 5.0, and 10.0 mg/mL hyaluronic acid or 0.05, 0.1, 0.5, and 1 mg/mL poly-L-lactic acid. After 24, 48, and 72 h, cells were detached using 0.05% trypsin and counted using a hemocytometer to determine the proliferation rate. Using a different set of plates and the same conditions as described above, cell viability was determined using a colorimetric test to assess cell metabolic activity (MTT test) at a wavelength of 590 nm. For accuracy, experiments were performed twice under the same conditions.
Quantification of type i collagen content by enzyme immunoassay
After 24, 48, and 72 h, the supernatant from cell cultures was collected and centrifuged at 5000 g for 15 min at 4 °C. Aliquots from each sample were tested by enzyme-linked immunosorbent assay (ELISA) to determine type I collagen content. The total amount of type I collagen was estimated in picograms per milliliter (pg/mL).
IN VIVO study. Preparation of samples for analysis
The in vivo study was performed on male Wistar rats. Before use, the poly-L-lactic acid-based filler was reconstituted by adding 10 ml of sterile water for injection, resulting in a final concentration of 15 mg/mL PLLA. The second filler was a hyaluronic acid solution with a concentration of 15 mg/mL. Animals were injected with a volume of 20 μL of the following preparations: HA, PLLA, phosphate-salt buffer (control for HA, CGC), or distilled sterile water (control for PLLA, CPLLA).
Fifteen rats were injected with different drugs on each side of the body or were left uninjected, i.e. five animals per each type of drug at each time point (15, 30, and 60 days). Each injection was made on the right or left side with a 27G gauge needle using a linear technique. Biopsy samples from the entire skin thickness were obtained from the treated areas immediately after euthanasia 15, 30 and 60 days later. Biopsy specimens were separated for histologic examination and Western blotting analysis.
Histological examination
For histologic examination, a tissue sample was taken from the back of each rat and fixed in 10% buffered neutral formalin solution. Sections were made in paraffin with a thickness of 4 μm, stained with hematoxylin and eosin and placed on slides in natural resin according to the standard technique. The distribution of filler biomaterial between collagen fibers was then quantified, and the presence of inflammatory cells was evaluated
Quantification of type I collagen by western blotting method
Skin samples were ground using nitrogen and homogenized with lysis buffer with protease inhibitors. The analysis data are presented as an index of total protein expression normalized for glyceraldehyde-3-phosphate dehydrogenase.
Results
IN VIVO study. Assessment of cell proliferation and viability
After 24 and 48 h, cell proliferation and viability were approximately the same in skin fibroblasts exposed to stabilized hyaluronic acid of different concentrations and in the control group. After 72 h, fibroblast proliferation was similar in fibroblasts treated with different concentrations of stabilized hyaluronic acid, but higher than in the control group. After 72 h, cell viability was similar in the control group and in skin fibroblast cultures that received 0.5 mg/mL HA, and higher than the same for cells that received HA at concentrations of 1 mg/mL, 5 mg/mL, and 10 mg/mL (Table 1).
TABLE. 1 Proliferation (A) and viability (B) of fibroblast cultures 24, 48, and 72 h after exposure to hyaluronic acid at different concentrations (0.5, 1.0, 5.0, and 10 mg/mL) compared with the control group.
| GROUP/WATCH | 24 | 48 | 72 |
| (A) | |||
| Control | 0,38 ± 0,03A, a | 0,52 ± 0,04A, b | 0,49 ± 0,05A, b |
| 0,5 mg/mL | 0,33 ± 0,07A, a | 0,56 ± 0,10A, b | 0,61 ± 0,06B, b |
| 1 mg/mL | 0,38 ± 0,09A, a | 0,60 ± 0,09A, b | 0,63 ± 0,14B, b |
| 5 mg/mL | 0,41 ± 0,05A, a | 0,53 ± 0,07A, b | 0,62 ± 0,03B, b |
| 10 mg/mL | 0,42 ± 0,07A, a | 0,58 ± 0,08A, b | 0,65 ± 0,05B, b |
| (B) | |||
| Control | 0,24 ± 0,04A, a | 0,32 ± 0,09A, b | 0,45 ± 0,14A, c |
| 0,5 mg/mL | 0,29 ± 0,07A, a | 0,29 ± 0,04A, a | 0,47 ± 0,13A, b |
| 1 mg/mL | 0,29 ± 0,07A, a | 0,32 ± 0,07A, a | 0,37 ± 0,07B, a |
| 5 mg/mL | 0,26 ± 0,06A, a | 0,32 ± 0,06A, a, b | 0,35 ± 0,10B, b |
| 10 mg/mL | 0,23 ± 0,02A, a | 0,29 ± 0,07A, a, b | 0,36 ± 0,09B, b |
Here and further in the tables, different capital letters indicate significant differences between groups for each stage of the experiment. Different lowercase letters indicate significant differences at different stages. Cell proliferation is expressed in cell number × 104 cells, viability is expressed in conventional units. Data are presented in the format “mean ± standard deviation”.
After 24 h, the proliferation of skin fibroblasts exposed to poly-L-lactic acid at concentrations of 0.05 mg/mL and 0.5 mg/mL was lower than in the other experimental groups. After 48 and 72 h, proliferation and viability were significantly reduced in fibroblasts that received PLLA of different concentrations compared with the control group. After 24, 48 and 72 h, cell viability was higher in the control group than in the groups where fibroblasts were exposed to PLLA at different concentrations (Table 2).
TABLE. 2 Proliferation (A) and viability (B) of fibroblast cultures 24, 48, and 72 h after treatment with poly-L-lactic acid at different concentrations (0.05, 0.1, 0.5, and 1 mg/mL) compared with the control group.
| GROUP/WATCH | 24 | 48 | 72 |
| (A) | |||
| Control | 0,47 ± 0,04A,C,a | 0,61 ± 0,07A,b | 0,64 ± 0,08A,b |
| 0,05 mg/mL | 0,26 ± 0,05B,a | 0,29 ± 0,06B,a | 0,30 ± 0,07B,a |
| 0,1 mg/mL | 0,40 ± 0,05C,D,a | 0,49 ± 0,05C,b | 0,48 ± 0,10C,b |
| 0,5 mg/mL | 0,34 ± 0,02D,a,c | 0,43 ± 0,03C,b | 0,40 ± 0,05D,b,c |
| 1 mg/mL | 0,46 ± 0,04C,a | 0,48 ± 0,05C,a | 0,48 ± 0,05C,E,a |
| (B) | |||
| Control | 0,29 ± 0,06A,D,a | 0,27 ± 0,02A,a | 0,40 ± 0,05A,b |
| 0,05 mg/mL | 0,16 ± 0,03B,a | 0,15 ± 0,04B,a | 0,29 ± 0,09B,b |
| 0,1 mg/mL | 0,24 ± 0,09C,a | 0,17 ± 0,07B,b | 0,27 ± 0,06B,a,c |
| 0,5 mg/mL | 0,23 ± 0,05C,a | 0,29 ± 0,06A,a | 0,28 ± 0,08B,a |
| 1 mg/mL | 0,32 ± 0,06D,a | 0,28 ± 0,05A,a | 0,31 ± 0,09B,a |
Quantification of type I collagen content
Immunoenzyme analysis showed that the culture medium of skin fibroblasts treated with stabilized hyaluronic acid and poly-L-lactic acid, regardless of concentration, contained more type I collagen compared to the control group at all stages of the experiment (Table 3).
TABLE. 3 Quantitative analysis of type I collagen content by enzyme immunoassay after 24, 48, and 72 h of treatment with hyaluronic acid (A) and poly-L-lactic acid (B) at different doses compared with the control group.
| GROUP/WATCH | 24 | 48 | 72 |
| (A) | |||
| Control | 15,59 ± 0,46A, a | 18,26 ± 1,39A, b | 16,16 ± 2,89A, a |
| 0,5 mg/mL | 28,90 ± 1,10B, a | 26,86 ± 1,08B, a | 27,45 ± 0,94B, a |
| 1 mg/mL | 27,76 ± 1,13B, a | 26,94 ± 1,26B, a | 25,02 ± 1,19C, a |
| 5 mg/mL | 24,95 ± 0,67C, a | 27,22 ± 0,44B, b | 22,82 ± 0,87D, c |
| 10 mg/mL | 24,24 ± 0,86C, a | 26,96 ± 0,81B, b | 21,53 ± 1,60D, c |
| (B) | |||
| Control | 15,59 ± 0,46A, a | 18,26 ± 1,39A, a | 16,16 ± 2,89A, a |
| 0,05 mg/mL | 21,09 ± 1,86B, a | 26,43 ± 1,28B, b | 25,10 ± 1,23B, b |
| 0,1 mg/mL | 21,51 ± 1,37B, a | 28,78 ± 1,19B, b | 28,34 ± 1,16B, b |
| 0,5 mg/mL | 23,15 ± 1,66B, C, a | 26,07 ± 2,89B, b | 27,73 ± 2,55B, b |
| 1 mg/mL | 25,30 ± 0,63C, D, a | 29,64 ± 1,20B, b, c | 27,44 ± 0,75B, a, c |
Results are expressed in pg/mL.
IN VIVO study. Histological examination
Characteristic images of skin biopsy specimens after different treatments are shown in (Figure 2, Figure 3, Figure 4). The presence of collagen fibers interspersed with hair follicles, sebaceous glands and blood vessels, characteristic of normal skin, was observed in both control groups (CGC and CPLLA) and at all stages of the experiment.

FIG. 2 Microphotographs of skin samples 15 days after injection of phosphate-salt buffer (control for hyaluronic acid: A and B), distilled water (control for poly-L-lactic acid: C and D), hyaluronic acid (E and F), and poly-L-lactic acid (G and H). Arrows indicate the presence of filler. Scale bar: A, C, E, and G = 500 μm; B, D, F, and H = 200 μm.
After 15 days, the greatest accumulation of hyaluronic acid (in the HA groups) was in the deep layers of the dermis (Fig. 2, E and F); however, after 30 and 60 days, the substance appeared evenly distributed (Fig. 3 and Fig. 4, E and F). No signs of inflammation were detected at any stage of the study.
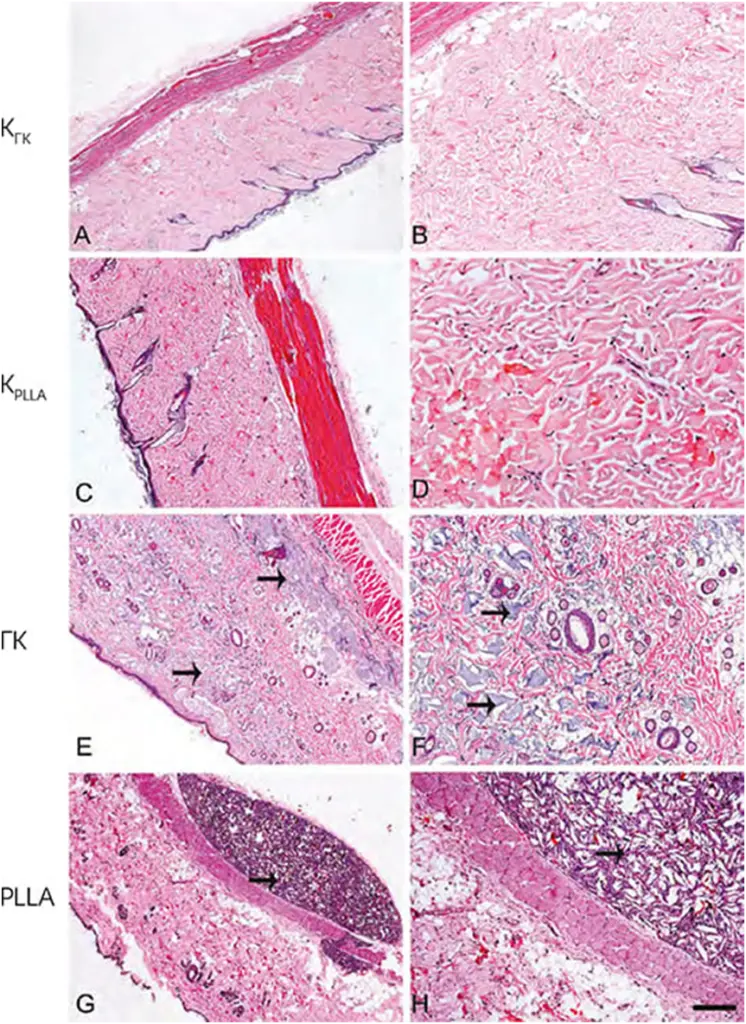
FIG. 3 Microphotographs of skin samples 30 days after injection of phosphate-salt buffer (A and B), distilled water (C and D), hyaluronic acid (E and F), and poly-L-lactic acid (G and H). Arrows indicate the presence of filler. Scale bar: A, C, E, and G = 500 μm; B, D, F, and H = 200 μm.
In contrast, the PLLA group showed an intense inflammatory process characterized by the presence of multinucleated cells, lymphocytes and macrophages, especially after 60 days. The breakdown of poly-L-lactic acid occurred over time, as evidenced by an increase in the number of giant multinucleated cells and areas of phagocytosis (Figure 2, Figure 3, Figure 4, G and H). In addition, numerous PLLA-derived filler crystals were observed, especially after 60 days.

FIG. 4 Microphotographs of skin samples 60 days after injection of phosphate-salt buffer (A and B), distilled water (C and D), hyaluronic acid (E and F), and poly-L-lactic acid (G and H). Arrows indicate the presence of filler. Asterisks indicate multinucleated giant cells (inset). Scale bar: A, C, E, and G = 500 μm; B, D, F, and H = 200 μm. The inset shows 20 μm.
Quantitative analysis of type I collagen content by western blotting method
Validation of the histologic analysis was provided by measuring the content of type I collagen by immunoblotting (Fig. 5). At all stages of the experiment, higher expression of type I collagen was observed when fillers were injected compared with the respective control groups (CGC or CPLLA). After 30 and 60 days, type I collagen expression was higher in the GC group than in the PLLA group.
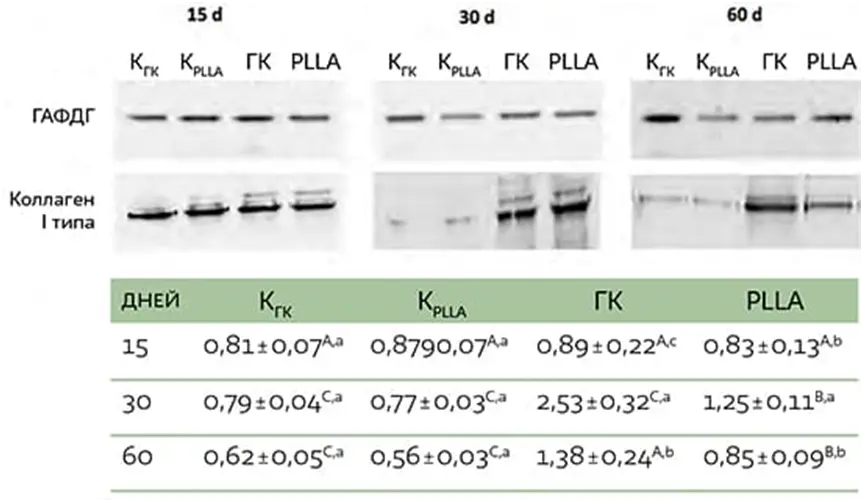
FIG. 5 Immunoblots of type I collagen expression levels in skin 15, 30, and 60 days after filler injection. Densitometric value (in conventional units, AU) normalizing protein content by glyceraldehyde-3-phosphate dehydrogenase (GAPDH). Different capital letters denote significant differences between groups at each stage of the experiment. Different lowercase letters denote significant differences between different stages of the experiment.
Discussion
Discussion
The results of the study confirm a decrease in fibroblast viability under the influence of poly-L-lactic acid in vitro and a negative effect on the extracellular matrix in vivo. In contrast, hyaluronic acid injections not only promote proliferation and high fibroblast viability, but also increase type I collagen without obvious signs of inflammation, in contrast to PLLA.
In fact, hyaluronic acid is a major component of the skin’s extracellular matrix, involved in regulating cell proliferation and migration [10], as well as promoting angiogenesis [15]. As a natural component of the skin, it is good because it does not cause rejection, has a low immunogenic effect, and also exhibits viscoelastic and hygroscopic properties, due to which it creates a moisturizing effect. In addition, hyaluronic acid has excellent mechanical properties and a moderate biodegradation period [3].
Thirty days after the start of the experiment, histological examination showed that HA was sometimes poorly distributed in the skin, and no inflammation was detected at any stage of the study, emphasizing its positive biological properties. In addition to promoting the production of type I collagen, hyaluronic acid also provides enhanced cell proliferation, which is consistent with the findings of Rock et al. [16].
These findings are consistent with earlier work that exogenous hyaluronic acid affects fibroblast proliferation and collagen synthesis, allowing for a predictable localized increase in volume.
In contrast to the promising results of the HA-based filler trials, the administration of a poly-L-lactic acid-based filler leads to a reduction in proliferation and cell viability in vitro, especially after 72 h. The results of histologic examination confirm that PLLA injections result in a local tissue reaction to the foreign body, which induces a strong inflammatory process generating many multinucleated cells surrounded by a fibrous capsule. After 60 days, the presence of PLLA filler in the tissue was still visible and accompanied by skin tension.
These data support the conclusion that dermal thickness in patients treated with poly-L-lactic acid filler is due to a local tissue response. Once the filler is injected, the tissue is mechanically stretched due to the introduction of fluid (some poly-L-lactic acid preparations are dissolved in large amounts of fluid before injection). Gradually, macrophages degrade PLLA into microspheres resulting from lactate formation [23], which in turn leads to the formation of many small crystals responsible for stimulating fibroblasts and type I collagen production [8, 24 – 26]. Poly-L-lactic acid particles have a flat pointed shape and dissolve unevenly in water, due to which accumulations of the drug may form [27]. In this regard, it is possible to observe clinically a shorter-term effect of HA than PLLA, which means that hyaluronic acid will need to be re-injected more often than poly-L-lactic acid.
Conclusion
Based on the results of the present study, we found that poly-L-lactic acid-based filler has a potentially unfavorable effect on the phenotype of fibroblasts. In contrast, hyaluronic acid has a positive effect on fibroblast phenotype in vitro and in vivo, including inducing more intensive cell proliferation and enhancing type I collagen synthesis.





